MHRA Opens Consultation on External Control Arms Using Real World Data
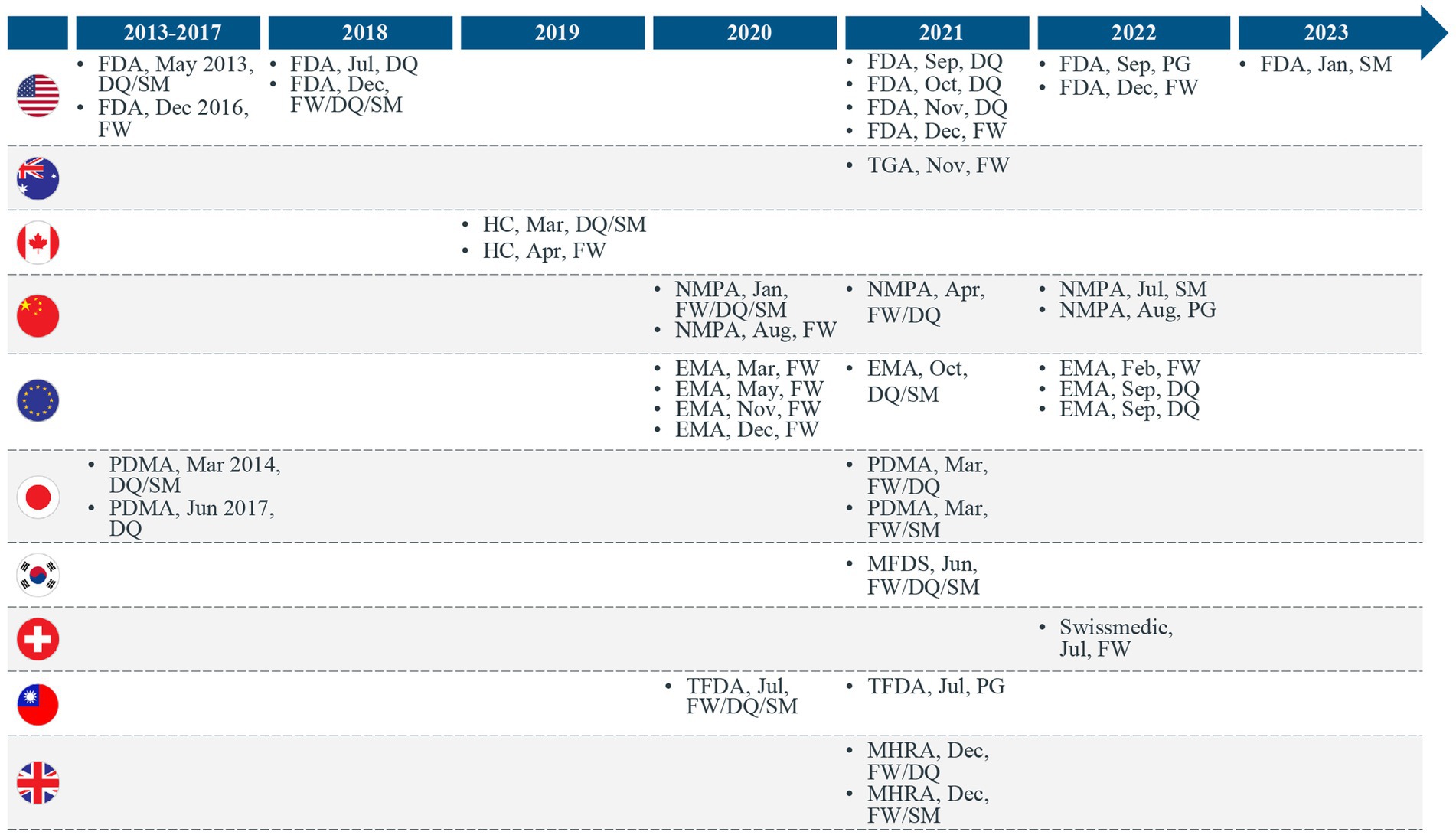
**MHRA Opens Consultation on External Control Arms Using Real World Data**
The Medicines and Healthcare products Regulatory Agency (MHRA), the United Kingdom’s regulatory body for medicines and medical devices, has recently opened a public consultation on the use of external control arms (ECAs) in clinical trials, leveraging real-world data (RWD). This move is part of MHRA’s ongoing efforts to modernize clinical trial methodologies and improve the efficiency and effectiveness of drug development processes.
**Understanding External Control Arms and Real-World Data**
External control arms are groups of patients used for comparison in clinical trials, who are not part of the randomized control group. Instead, these patients are sourced from existing datasets, such as electronic health records, patient registries, or other real-world data sources. Real-world data refers to health information collected outside of traditional clinical trials, often reflecting a broader patient population and more diverse clinical settings.
The integration of ECAs using RWD into clinical trials presents an opportunity to enhance the relevance and applicability of trial outcomes. By utilizing data from real-world settings, researchers can potentially reduce the need for placebo groups, accelerate patient recruitment, and provide insights into how treatments perform across different populations.
**The Consultation Process**
The MHRA’s consultation seeks input from a broad range of stakeholders, including pharmaceutical companies, healthcare professionals, academic researchers, and patient advocacy groups. The objective is to gather diverse perspectives on the potential benefits, challenges, and ethical considerations associated with using ECAs and RWD in clinical trials.
Key areas of focus in the consultation include:
1. **Methodological Considerations**: Evaluating the statistical and methodological approaches for integrating ECAs into clinical trials. This includes discussions on data quality, selection bias, and the comparability of real-world data to traditional randomized controlled trial data.
2. **Regulatory Framework**: Exploring how existing regulatory guidelines can be adapted to accommodate the use of ECAs and RWD. The consultation aims to identify any regulatory barriers and propose solutions to facilitate the adoption of these innovative approaches.
3. **Ethical and Patient Considerations**: Addressing ethical concerns related to patient privacy, data security, and informed consent. The consultation also seeks to understand patient perspectives on the use of their health data in clinical research.
4. **Impact on Drug Development**: Assessing how the integration of ECAs and RWD could influence the speed, cost, and success rates of drug development. The consultation will explore potential impacts on market access and reimbursement decisions.
**Potential Benefits and Challenges**
The use of ECAs and RWD in clinical trials offers several potential benefits. It can lead to more efficient trial designs, reduce the burden on patients, and provide a more comprehensive understanding of a drug’s performance in the real world. Additionally, it may facilitate the study of rare diseases or conditions where traditional trials are challenging to conduct.
However, there are also significant challenges to address. Ensuring the quality and reliability of real-world data is paramount, as is maintaining the integrity of trial results. The consultation aims to identify strategies to overcome these challenges and establish best practices for the use of ECAs.
**Conclusion**
The MHRA’s consultation on external control arms using real-world data marks a significant step towards modernizing clinical trial methodologies. By engaging with stakeholders and exploring innovative approaches, the MHRA aims to enhance the efficiency and relevance of clinical research. The outcomes of this consultation could pave the way for more adaptive and patient-centered clinical trials, ultimately benefiting patients and healthcare systems alike.
Stakeholders are encouraged to participate in the consultation process and contribute their insights and expertise. The MHRA’s initiative represents an important opportunity to shape the future of clinical trials and ensure that regulatory frameworks keep pace with advancements in data science and healthcare innovation.