Did the European Parliament Miss the Opportunity to Enhance Orphan Drug Development?
**Did the European Parliament Miss the Opportunity to Enhance Orphan Drug Development?**
The European Union (EU) has long been a global leader in the regulation and promotion of orphan drugs—medicines developed to treat rare diseases, which often affect small patient populations. The Orphan Drug Regulation, introduced in 2000, was a pioneering piece of legislation aimed at incentivizing pharmaceutical companies to invest in the development of treatments for rare diseases. However, as the landscape of drug development evolves and the needs of patients with rare diseases become more complex, there is growing concern that the European Parliament may have missed a crucial opportunity to further enhance orphan drug development.
### The Current State of Orphan Drug Development in the EU
The Orphan Drug Regulation has undoubtedly been successful in stimulating the development of treatments for rare diseases. Since its implementation, over 2,000 orphan designations have been granted, and more than 200 orphan drugs have been authorized for marketing in the EU. These drugs have provided new hope for patients suffering from conditions that were previously neglected by the pharmaceutical industry.
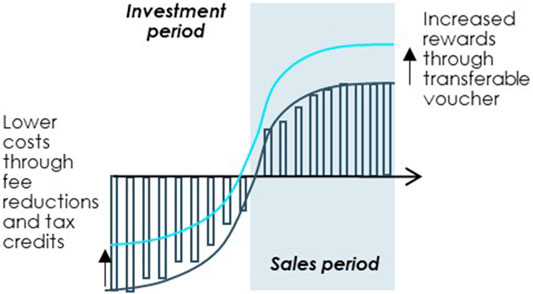
The regulation offers several incentives to companies developing orphan drugs, including market exclusivity for 10 years, fee reductions, and access to scientific advice from the European Medicines Agency (EMA). These incentives have been instrumental in encouraging investment in the orphan drug sector, which is often seen as high-risk due to the small patient populations and the challenges associated with developing treatments for rare diseases.
### The Need for Reform
Despite the successes of the Orphan Drug Regulation, there are growing calls for reform. Critics argue that the current framework does not adequately address the evolving needs of patients with rare diseases, nor does it fully capitalize on the potential of new scientific and technological advancements.
One of the key issues is the definition of “orphan” status, which is granted to drugs intended to treat diseases affecting fewer than 5 in 10,000 people in the EU. This threshold has been criticized for being too rigid, potentially excluding diseases that, while rare, still represent significant unmet medical needs. Additionally, some stakeholders argue that the 10-year market exclusivity period may not be sufficient to incentivize the development of treatments for ultra-rare diseases, where the patient population is even smaller.
Another concern is the lack of alignment between the Orphan Drug Regulation and other EU policies, such as those related to pricing and reimbursement. The high cost of orphan drugs has led to disparities in access across EU member states, with some countries unable to afford these treatments. This has raised questions about the sustainability of the current model and the need for a more coordinated approach to pricing and reimbursement.
### Missed Opportunities for Enhancement
In recent years, there have been several opportunities for the European Parliament to address these issues and enhance the Orphan Drug Regulation. However, many stakeholders believe that these opportunities have not been fully seized.
For example, the European Commission launched a public consultation on the Orphan Drug Regulation in 2019, seeking input on potential revisions to the legislation. While the consultation highlighted the need for reform, the subsequent proposals have been criticized for being too conservative and failing to address the most pressing challenges facing the orphan drug sector.
Moreover, the European Parliament has been slow to act on recommendations from patient advocacy groups, industry stakeholders, and experts in the field. These recommendations have included calls for greater flexibility in the definition of orphan status, more robust incentives for the development of treatments for ultra-rare diseases, and improved coordination between the Orphan Drug Regulation and other EU policies.
### The Path Forward
While the European Parliament may have missed some opportunities to enhance orphan drug development, there is still hope for meaningful reform. The ongoing review of the Orphan Drug Regulation provides a chance to address the shortcomings of the current framework and ensure that it remains fit for purpose in the face of new challenges and opportunities.
To achieve this, the European Parliament must take a more proactive approach, engaging with a wide range of stakeholders and considering bold and innovative solutions. This could include revising the definition of orphan status to better reflect the needs of patients with rare diseases, extending market exclusivity for ultra-rare conditions, and developing a more coordinated approach to pricing and reimbursement.
Additionally, the European Parliament should explore ways to leverage new scientific and technological advancements, such as gene therapies and personalized medicine, to drive innovation in the orphan drug sector. By doing so, the EU can continue to be a global leader in the development of treatments for rare diseases and ensure that patients across Europe have access to the life-saving medicines they need.
### Conclusion
The European Parliament has played a crucial role in shaping the orphan drug landscape in the EU, but there is a growing consensus that more needs to be done to enhance orphan drug development. While some opportunities for reform may have been missed, there is still time to make meaningful changes that will benefit patients with rare diseases. By taking a more proactive and innovative approach, the European Parliament can ensure that the EU remains at the forefront of orphan drug development and that